The immune system plays an important role in keeping us healthy. But an overactive immune response can lead to excess inflammation, characterized by an increase in fluids, immune cells, and other substances in the affected area. Inflammation is part of the body’s natural response to infection or wounds, but chronic inflammation can lead to a number of health diseases, including cancer.
Memorial Sloan Kettering cell biologist Philipp Niethammer is using advanced imaging approaches to investigate inflammation and how the immune system responds to threats. We spoke with Dr. Niethammer recently about his research.
What aspects of inflammation is your lab focusing on?
We’re generally interested in how damage to the body’s tissues initiates inflammatory and tissue-repair reactions. These types of reactions involve the migration of white blood cells to the site of tissue damage.
When the body encounters an infectious agent, such as bacteria or a virus, the immune system is essential in fighting it off. Inflammation is important for the body’s healing response. But very often inflammation can lead to chronic diseases of the epithelium [the tissues that line the cavities and surfaces of the body], such as asthma and Crohn’s disease.
We want to know what kinds of molecular signals guide the migration of immune cells and initiate these not-always-wanted responses.
What happens when inflammation goes wrong?
Some immune cells are professional killers. They secrete chemicals that are supposed to kill bacteria, but they also can damage the body’s own cells.
Because of that, inflammatory responses have to be really tightly regulated. This is the big magic that our bodies do: When they work the way they’re supposed to, they recruit just the right number of immune cells that are needed to heal a wound.
Many molecular pathways influence the way immune cells migrate to injury sites. Sometimes the pathways are overactivated — too many immune cells are recruited and we get too much inflammation. And sometimes the pathways are not functional at all, which leads to immune deficiencies or infections.
How does this relate to cancer?
If a person has a chronic influx of white blood cells into certain tissues — like the gut, for example — this lasting inflammatory reaction can promote the development of tumors. In part this is because the toxic chemicals that are released by our white blood cells not only kill pathogens as they are supposed to but also unfortunately damage and mutate our own cells, which may enable tumors to form.
In fact, we know that inflammatory conditions of the gastrointestinal tract such as Crohn’s disease and colitis increase the incidence of cancers in those organs. Acid reflux disease is another example: When stomach acid persistently injures the epithelial lining of the esophagus, white blood cells are constantly recruited to the site of damage to release toxic antimicrobial chemicals. If this goes on unchecked for a long time, esophageal cancer can result.
How do you go about studying the immune response?
What we want to know is, how does the body know when an injury or infection has occurred? How does it detect and respond to tissue damage, and how might we be able to interfere with that process when it is undesired?
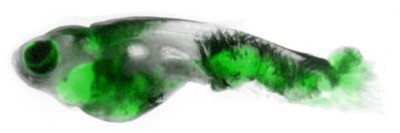
The body’s response to healing wounds has been evolutionarily conserved in the animal kingdom, which means we can learn about the basic mechanisms of the response by studying simple animals, and these discoveries will be relevant to human biology. The organism that we’re using in my lab is the zebrafish.
Zebrafish larvae are transparent and very small, which makes them an ideal animal model to study under the microscope. What makes them very useful is that they have a very simple structure in their tail fins that is basically two layers of epithelial cells.
We anesthetize the larvae, put them under the microscope, and use a laser or a tiny needle to poke a hole in the outer layer of the epithelium. The different types of immune cells are labeled with dyes that glow in different colors when we shine fluorescent light on them. Then we can observe the cells’ movements after we poke the hole. We measure how fast they move toward the wound, and whether they go straight there or probe around first. Do they know where the wound is, or do they end up there by chance?
What can you learn from doing this?
The wounds we make are very small, non-bleeding wounds. We think they accurately represent the small mechanical and chemical stresses that happen in our bodies on a day-to-day basis or during disease — for example, tiny tears in the lungs that might happen when we cough or inhale small particles, or tiny tears in the gut that are part of normal digestive processes.
We are able to see the first brigade of immune cells that arrives to disinfect the wound. Usually these are neutrophils, a very common type of white blood cell. And we can look at what signals are required for the epithelial cells to close up the hole at the wound site so that no more pathogens can enter.
What’s the ultimate goal of your research?
Once we know what pathways are triggering the chain reaction of the body’s defense system, we’re hoping to find ways to modulate these pathways that could be beneficial for the treatment of particular diseases. Depending on the condition, we may want to increase or decrease the response.
We’re not working on one particular side of this pathway. We’re really interested in the logic of the body’s entire defense system.
Describe your role as a basic scientist working at a cancer center.
For me the biggest challenge has always been to understand why the body reacts the way it reacts, not only to interfere with certain responses but also to gain a broader understanding of these processes. Presumably that will lead to new ways to treat different conditions, whether cancer or other kinds of diseases.