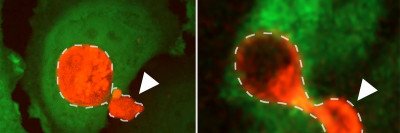
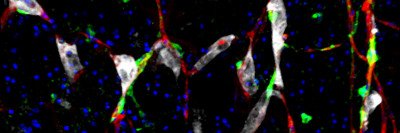
Some cells are more vulnerable than others to a type of iron-dependent death called ferroptosis.
Cells can die in several ways. One such method, discovered only within the past decade, is ferroptosis — literally, death by iron. This type of death requires iron and is linked to a cell’s use of oxygen for metabolism.
Ferroptosis is a main cause of ischemic heart disease, a condition caused by the death of cells in the heart muscle. But it likely serves other purposes too, like preventing cancer formation. Cells that might form tumors are instead killed by ferroptosis triggered by working tumor suppressor proteins.
Intriguingly, cancer cells that have spread, or metastasized, are more sensitive than normal cells to ferroptosis, a vulnerability that scientists seek to exploit.
“Ferroptosis is becoming a hot topic among pharmaceutical companies,” says Xuejun Jiang, a cell biologist in the Sloan Kettering Institute whose lab is seeking to understand how ferroptosis works. “The idea is that if you can induce ferroptosis in a controlled manner, it might be a great way to treat cancer.”
In a recent study, published July 24 in the journal Nature, Dr. Jiang and colleagues, including two of his former postdoctoral fellows who are now professors in China, found that whether cells are vulnerable to ferroptosis or not depends on signals initiated by neighboring cells. When present, these signals guard against ferroptosis.
The discovery sheds light on why metastatic cancer cells, which have lost these connections, are especially vulnerable to this type of cell death. It also suggests a new treatment strategy.
Good Contacts Make Good Neighbors
Neighboring cells relay signals by way of proteins called cadherins, which respond to physical contact. Dr. Jiang’s team discovered that these signals suppress the actions of a protein called YAP, which promotes ferroptosis. When cells lose contact with their neighbors, YAP is no longer suppressed and ferroptosis can be more easily triggered.
As cancer cells become more aggressive, they often undergo what is called an epithelial-mesenchymal transition (EMT). This means that they lose the adhesive properties typical of epithelial cells and become more mobile and invasive. The spread of cancer through metastasis is the main way that cancer kills.
But metastatic cells also lose the signals provided by cadherins when they are no longer stuck to their neighbors, which makes them vulnerable to ferroptosis.
A Mesothelioma Weak Point
The finding has particular relevance to mesothelioma. This aggressive type of cancer affects the lining of internal organs and tends to spread quickly. Mesothelioma cells often contain a mutation in a protein called Merlin, or NF2, which is part of the chain of signals that begins with cadherins and ends with YAP. The mutation inactivates Merlin and interrupts this signaling chain. Using a mouse model, Dr. Jiang and colleagues showed that mesothelioma tumors with this mutation are very sensitive to ferroptosis.
“Mutations in Merlin and other tumor suppressor proteins in this signaling process usually make cancer cells more malignant and harder to treat,” Dr. Jiang says. “But on the other hand, these events also make cancer cells more susceptible to ferroptosis, which could be their Achilles’ heel.”
These mutations occur in many types of cancer besides mesothelioma, he points out. Potentially, they could serve as biomarkers to predict responsiveness to ferroptosis-inducing therapies.
Currently, there are no drugs approved by the US Food and Drug Administration that specifically promote cancer cell ferroptosis. Many research groups and pharmaceutical companies are working on developing them.