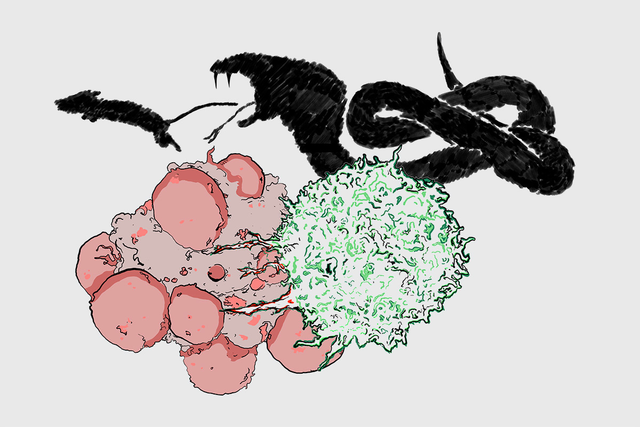
The way that a T cell (green) kills a cancer cell (red) is similar in some ways to how a snake kills its prey, with toxic venom. Image credit: Miguel De Jesus
A few years ago, immunologist Morgan Huse made a startling discovery that would set his research down an uncharted path.
He was investigating how the immune cells called T cells kill their targets — often pathogens, sometimes cancer cells. Typically, T cells kill by releasing toxic chemicals that cause the target to self-destruct. But T cells with a particular genetic mutation were less able to kill, he found. This was the case even though the cells had all of the necessary equipment.
What was different about these cells, he learned, was that they exerted less force against their target. They lacked the muscle to pack a punch.
This was the first time that anyone had shown that generating force is a crucial component of how T cells kill. For Dr. Huse, a member of the Sloan Kettering Institute Immunology Program, it was the eye-opener that led him to refocus his lab’s research efforts.
“Force has been neglected as a feature of cellular life,” Dr. Huse says. “The reason we know so much about biochemistry and molecular biology is that we can literally grind up cells and do reactions in test tubes. It’s much more difficult to observe and measure physical processes at a cellular level.”
It is difficult, but not impossible. With sensitive tools and a new angle of vision, Dr. Huse and his colleagues are now bringing force to the fore. In the process, they’re rattling scientists’ understanding of some fundamental concepts.
Force Be with You
The textbook view of how T cells kill is that they essentially lock horns with a target cell (using a special receptor) and then release a toxic payload of a molecule called perforin, which literally pokes holes in the cell and causes it to self-destruct. It’s considered a chemical process. But when Dr. Huse and his colleagues started looking at the architecture of this meeting point — called the immunological synapse — it became clear that quite a bit more was going on.
T cells don’t just latch on to a target cell with their receptors. They actually extend fingerlike projections into the target cell’s membrane. And it’s at the site of these projections that they release perforin.
“We think of these T cell protrusions as kind of like the fangs of a snake,” Dr. Huse says. “The purpose of fangs is to position venom in the appropriate place beneath the skin of animal prey. That effectively allows the snake to kill more prey with less venom. T cells are more or less doing the same thing. They are using these physical structures to maximize the killing power of their venom.”
By applying pressure at precise points, he explains, these cellular “fangs” may make it more likely that a hole will form where perforin is released.
“Kind of like when you want to puncture a partially deflated balloon with a needle, it helps to squeeze the balloon first,” Dr. Huse says.
Tools of the Trade
The idea that a cell’s shape plays a key role in its function is well accepted in biology. The shape of neurons and epithelial cells, for example, helps them transmit electrical signals and absorb nutrients, respectively. What is not as well appreciated is that, when specific cellular structures move, they can transmit forces that add a whole new dimension to their functionality.
“Think of a drill bit,” Dr. Huse says. “You can’t really understand how it works by looking at a still picture, but when you see it in motion, it becomes pretty obvious.”
Measuring the forces generated by a cell’s moving parts requires exquisitely sensitive instruments. One approach uses a glass pipette (a tool somewhat like an eyedropper) that has been stretched to no more than a few micrometers in diameter. That’s a fraction of the width of a human hair. The pipette is flexible, and how much it bends depends on how much force is applied. The scientists attach a bead coated with target molecules to the end of the pipette. Then they present the bead to a T cell, which grabs onto it and either pushes or pulls the pipette. The scientists can measure how much the pipette moves and calculate the corresponding pushing or pulling force.
Another tool they use is an array of flexible plastic micropillars, similar to tiny bristles on a microscopic hairbrush. The micropillars are coated with target molecules. When a T cell lands on the array, it attacks the micropillars as if they were a target cell. This action moves the pillars in a particular direction. Each of these movements corresponds to a force exerted.
This system has much higher spatial resolution than the pipette approach and has enabled the scientists to identify specific hot spots of forces in the immune synapse.
Measuring T Cell Forces with a Micropillar Array
Factoring Forces In
More and more, scientists are learning that physical forces play an important role in many biological processes. There’s even a connection to cancer.
As other scientists at SKI have shown, physical forces allow cancer cells to colonize other parts of the body, forming metastatic tumors. To successfully grow in a new location, a cancer cell must stiffen and grow along blood vessels in this unfamiliar niche. This stiffening trips molecular sensors in the cancer cell that signal it to start dividing.
The Huse lab is interested in exploring whether the stiffness of a target — either a cancer cell or a pathogen — can influence immune-cell-mediated killing.
“Understanding how cancer cells, pathogens, and immune cells depend on different forces enables us to think up ways to blunt or assist those forces, like practicing cellular judo,” Dr. Huse says.


