SKI immunologist Gretchen Diehl
The immune system’s main job is identifying things that can make us sick. In the language of immunology, this means distinguishing “self” from “non-self”: The cells of our organs are self, while disease-causing bacteria and viruses are non-self.
But what about the billions of bacteria that live in our guts and provide us with benefits like digesting food and making vitamins? Are they friend or foe?
This isn’t only a philosophical question. An immune system that mistakes our good gut bacteria for an enemy can cause a dangerous type of inflammation in the intestines called colitis. An immune system that looks the other way while gut microbes spill past their assigned borders is equally dangerous. Understanding how the immune system learns to make a brokered peace with its microbial residents, called the microbiota, is therefore an important area of research.
“Right now, each of us has immune cells in our body that can recognize and attack specific members of our gut microbiota,” says Gretchen Diehl, an immunologist in the Sloan Kettering Institute. “So, it’s a puzzle why more of us don’t have colitis caused by these cells attacking our gut microbes.”
To try to solve that puzzle, Dr. Diehl and her colleagues, including SKI postdoctoral researcher Daniel Zegarra-Ruiz and immunologist Matthew Bettini at the University of Utah, recently conducted a study using lab mice to explore what happens to microbe-specific T cells (a type of immune cell) when mice are exposed as young pups to a common gut microbe.
“We thought that maybe T cells specific for this microbe would be eliminated from the mice, or perhaps the mice would develop anti-inflammatory T cells that would protect them from developing colitis,” Dr. Diehl says. In other words, they hypothesized the bacteria would be seen as self.
Surprisingly, that wasn’t the case. Not only were the microbe-specific T cells not deleted, they actually grew in number. The results, which were published May 12, 2021, in Nature, document a previously unknown way that gut microbes interact with the immune system and raise yet more questions about what goes awry in autoimmune disease.
Hidden Highway
The scientists focused their attention on an immune system organ called the thymus, which is located in the upper chest behind the breastbone. The thymus’s main role is “educating” T cells about which markers, or antigens, in the body are self versus non-self. T cells that recognize self are actively culled while those that do not recognize self are spared. The non-self-recognizing T cells are then released into the circulation where they patrol for viruses, bacteria, and other invaders.
The education process is thought to be primarily about protecting the body from self-attacking T cells that could cause trouble in the form of autoimmunity. Dr. Diehl and her colleagues were taken aback to find that rather than eliminating T cells that recognize gut bacteria, the thymus was giving them the green light.
“We were very, very surprised that when we colonized mice with gut bacteria, instead of seeing the development of regulatory T cells that calm immune reactions or loss of microbe-specific T cells, we saw an expansion of them,” she says. “As far as we are aware, this is the first time anyone has shown the thymus playing this role in the expansion of microbe-specific T cells.”
Next they asked: How is information about gut bacteria making its way to the thymus?
Using standard gut microbiota detection techniques, they could see that bacterial DNA was showing up in the thymus, suggesting that the gut is somehow in communication with this organ via DNA. But that only raised the further question of how this DNA was getting there.
One possible explanation is that bacterial DNA is carried there by immune cells called dendritic cells, whose main job is, in fact, to carry suspicious antigens from tissues to lymph nodes. Traveling from the gut to the thymus hadn’t been seen, but the scientists couldn’t rule out that possibility.
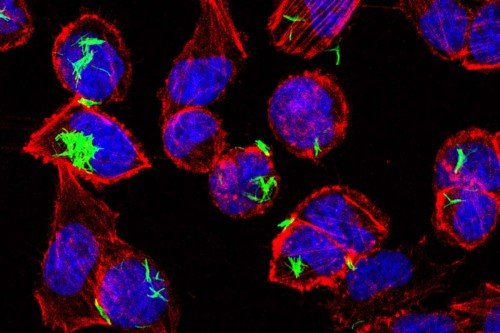
To track the movement of dendritic cells, Dr. Diehl and her colleagues took advantage of a hi-tech lab mouse developed by Kat Hadjantonakis’s lab at MSK. The mouse had been engineered to make a fluorescent marker called green fluorescent protein (GFP) in its cells. When GFP-containing cells are hit with light from a laser, they change from emitting green fluorescence to emitting red. By shining the laser in the intestine of the mouse, the scientists could turn gut dendritic cells red and then watch and see if red cells turned up in the mouse thymus. Sure enough, they did.
“The dendritic cells are clearly migrating that whole way, which is kind of crazy,” Dr. Diehl says.
A Temporary Window
The gut-to-thymus journey only happens in very young animals. When the scientists looked at older animals, they didn’t see the red cells turning up. Nor did they find any gut microbe DNA in the thymus or expansion of gut-specific T cells.
What might be the purpose of this gut-to-thymus traffic that only happens during a specific developmental window in the mice?
“What we think is happening is a kind of templating on the immune system,” Dr. Diehl says. “In that timeframe, the mouse immune system is very underdeveloped and the most relevant thing for it to recognize is microbes. So, it brings gut antigens to the thymus to educate the T cells about these and related dangers.”
As evidence for this possibility, they showed that the T cells that recognize the introduced bacteria also afford protection against pathogens the mice hadn’t yet seen. But because they recognize gut microbes, these T cells can also cause inflammation that can lead to colitis.
What the scientists next want to understand is whether this process differs in people who are more susceptible to colitis.
Dr. Diehl wonders: “Do you have this happening for a more extended period of time in people with colitis? Do you have it happening for less? Does it restart in someone with colitis? These are all questions we want to explore.”