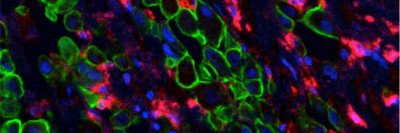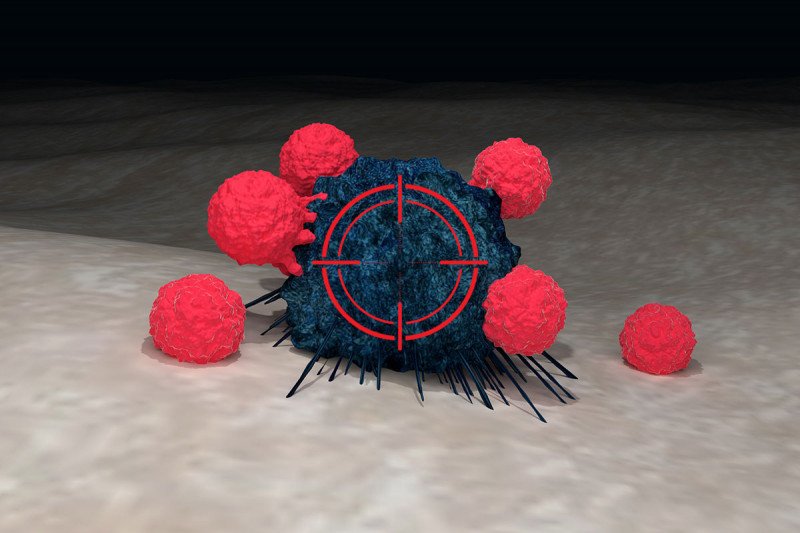
T cells (red) can be genetically modified to recognize cancer cells (blue) with certain characteristics. Radiation therapy (represented by crosshairs) may help these engineered immune cells kill more tumor cells.
For people with certain leukemias and lymphomas, an immunotherapy with chimeric antigen receptor (CAR) T cells has proven to be potentially lifesaving. The US Food and Drug Administration approved two such therapies in 2017.
The approach’s success in treating these cancers stems, in part, from the presence of a clear target: a molecule on the surface of blood cancer cells called CD19. CAR T cells outfitted with a protein that latches on to CD19 can easily find and destroy these cancer cells.
CD19 flags nearly all blood cancer cells, but such a prime target is hard to come by in other cancers. This makes it challenging to extend the benefits of CAR therapy to other cancer types, including solid tumors, like lung cancer, breast cancer, and pancreatic cancer.
One way to address this problem, new research from MSK suggests, is by combining CAR therapy with radiation therapy.
“We found that radiation made it easier for CAR T cells to kill tumor cells, including tumor cells that that did not express the CAR target and would otherwise escape from the CAR therapy,” says Michel Sadelain, Director of the Center for Cell Engineering at MSK and the corresponding author on a new paper published on October 25 in Molecular Therapy. “We made this discovery in a mouse model of pancreatic cancer, but the approach is applicable to any cancer in principle.”
Not What They Expected from Adding Radiation
Pancreatic ductal adenocarcinoma (PDAC) is a deadly cancer that is currently hard to treat with anything other than surgery. CAR therapy has so far not been effective. In part, that’s because the tumor cells are not all alike with respect to potential CAR targets. Although some cells have markers that could be targets for CARs, not all cells have them, making it difficult to design a CAR that would work against every cancer cell in the tumor.
One marker that is found intermittently in pancreatic cancer is called sialyl Lewis-A (sLeA). The researchers wondered if radiation might spur more of the PDAC cells to express, or make, this marker.
They gave mice low-dose radiation before administering the sLeA-specific CARs. They found that the combination of radiation and CARs did indeed increase the amount of tumor that was destroyed but not in the way they had initially hypothesized. That is, the number of cells making sLeA — the CAR target — did not change.
“That’s when we knew that some other mechanism of killing must be involved,” Dr. Sadelain says.
On the TRAIL of a New CAR T Role
Carl DeSelm, a physician-scientist working in Dr. Sadelain’s laboratory, analyzed what genes were turned on in the cells before and after low-dose radiation. He used a technique called RNAseq, which measures messenger RNA transcribed from genes. He found that radiation triggered the cells to turn on genes related to a kind of programmed cell death. The process is initiated by a protein called TRAIL (short for tumor necrosis factor–related apoptosis-inducing ligand). When TRAIL is present in the area around cells that have these genes turned on, those cells are cued to self-destruct.
Next Dr. DeSelm wanted to find out if CAR T cells produce TRAIL, so he analyzed RNA and protein produced by these cells in the presence and absence of a tumor. He found that TRAIL production goes way up when the CAR T cells bind to the sLeA target.
And here’s where it gets really interesting. The combination of radiation and CARs resulted in the death of pancreatic tumor cells that have the sLeA target as well as those that do not.
A Single Human Case: A Person with Lymphoma
Researchers won’t know for sure whether the same holds true in people with cancer until they conduct a clinical trial, which is currently being planned. But one known case report gives them hope. A person with lymphoma who was to receive CD19 CAR therapy had noncurative local radiation to his leg first, to relieve pain resulting from the disease.
Weeks later, after initially subsiding, the disease rebounded in various spots around his body. But it did not grow in the area that was irradiated. This result suggests — although it does not prove — that the radiation sensitized the tumor cells in his leg to TRAIL-mediated CAR T killing.
“Radiation therapy is currently used at some point in the treatment of about half of people with cancer that has spread, and for others it is commonly employed to improve tumor control in nearly all types of cancer,” says Dr. DeSelm, who is now an assistant professor of radiation oncology at Washington University in St. Louis. “Our hope is that CAR therapy might provide people with an added benefit when integrated with these standard radiation procedures.”
MSK medical oncologist Lia Palomba and radiation oncologist Joachim Yahalom, also authors on the paper, will be leading a clinical trial to test this approach.